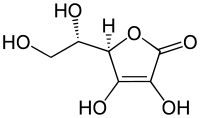
Ascorbic acid
| L-Ascorbic acid | |
|---|---|
 |
|
 |
|
|
(5R)-[(1S)-1,2-dihydroxyethyl]-3,4-dihydroxyfuran-2(5H)-one
|
|
|
Other names
Vitamin C
|
|
| Identifiers | |
| CAS number | 50-81-7 |
| PubChem | 5785 |
| ChemSpider | 17206850 |
| EC number | 200-066-2 |
| ATC code | A11 |
|
SMILES
OC=1C(OC(=O)C=1O)[C@@H](O)CO
|
|
|
InChI
InChI=1/C6H8O6/c7-1-2(8)5-3(9)4(10)6(11)12-5/h2,5,7-10H,1H2/t2-,5?/m0/s1
Key: CIWBSHSKHKDKBQ-SZSCBOSDBY |
|
| Properties | |
| Molecular formula | C6H8O6 |
| Molar mass | 176.12 g mol−1 |
| Appearance | White or light yellow solid |
| Density | 1.65 g/cm3 |
| Melting point |
190-192 °C, 463-465 K, 374-378 °F (decomp.) |
| Solubility in water | 33 g/100 ml |
| Solubility in ethanol | 2 g/100 ml |
| Solubility in glycerol | 1 g/100 ml |
| Solubility in propylene glycol | 5 g/100 ml |
| Solubility in [[{{{Solvent4}}}]] | insoluble in diethyl ether, chloroform, benzene, petroleum ether, oils, fats, fat solvents |
| Acidity (pKa) | 4.10 (first), 11.6 (second) |
| Hazards | |
| MSDS | JT Baker |
| LD50 | 11.9 g/kg (oral, rat)[1] |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
Ascorbic acid is a sugar acid with antioxidant properties. Its appearance is white to light-yellow crystals or powder, and it is water-soluble. One form of ascorbic acid is commonly known as vitamin C. The name is derived from a- (meaning "no") and scorbutus (scurvy), the disease caused by a deficiency of vitamin C. In 1937 the Nobel Prize for chemistry was awarded to Walter Haworth for his work in determining the structure of ascorbic acid (shared with Paul Karrer, who received his award for work on vitamins), and the prize for Physiology or Medicine that year went to Albert Szent-Györgyi for his studies of the biological functions of L-ascorbic acid. At the time of its discovery in the 1920s, it was called hexuronic acid by some researchers.[2]
Contents |
Chemistry
Acidity
Ascorbic acid behaves as a vinylogous carboxylic acid where the electrons in the double bond, hydroxyl group lone pair, and the carbonyl double bond form a conjugated system. Because the two major resonance structures stabilize the deprotonated conjugate base of ascorbic acid, the hydroxyl group in ascorbic acid is much more acidic than typical hydroxyl groups. In other words, ascorbic acid can be considered an enol where the deprotonated form is a stabilized enolate.
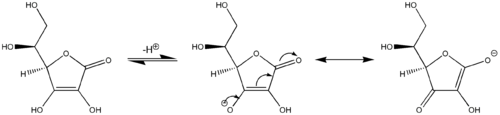
Tautomerism
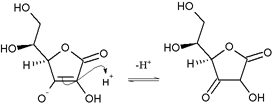
Ascorbic acid also interconverts into two unstable ketone tautomers by proton transfer, although it is the most stable in the enol form. The proton of the hydroxyl of the enol is removed. Then a pair of electrons from the resulting oxide anion pushes down to form the ketone at the 2 or 3 position and the electrons from the double bond move to the 3 or 2 position, respectively, forming the carbanion, which picks up the proton resulting in two possible forms: 1-carboxyl-2-ketone and 1-carboxyl-3-ketone.
Determination
The concentration of a solution of ascorbic acid can be determined in many ways, the most common ways involving titration with an oxidizing agent.
- Iodine
Another method involves using iodine and a starch indicator, wherein iodine reacts with ascorbic acid, and, when all the ascorbic acid has reacted, the iodine is then in excess, forming a blue-black complex with the starch indicator. This indicates the end-point of the titration. As an alternative, ascorbic acid can be reacted with iodine in excess, followed by back titration with sodium thiosulfate while using starch as an indicator.[3]
- Iodate and iodine
The above method involving iodine requires making up and standardising the iodine solution. One way around this is to generate the iodine in the presence of the ascorbic acid by the reaction of iodate and iodide ion in acid solution, the ionic equation for this reaction follows;
- IO3− + 5 I− + 6 H+ → 3 I2 + 3 H2O
- N-Bromosuccinimide
A much-less-common oxidising agent is N-bromosuccinimide, (NBS). In this titration, the NBS oxidises the ascorbic acid (in the presence of potassium iodide and starch). When the NBS is in excess (i.e., the reaction is complete), the NBS liberates the iodine from the potassium iodide, which then forms the blue/black complex with starch, indicating the end-point of the titration.
Antioxidant mechanism
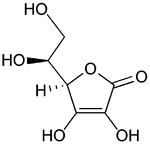 |
 |
(reduced form of Vitamin C)
Bottom: dehydroascorbic acid
(oxidized form of Vitamin C)
Ascorbate usually acts as an antioxidant by being available for energetically favourable oxidation. Many oxidants (typically, reactive oxygen species) such as the hydroxyl radical (formed from hydrogen peroxide), contain an unpaired electron, and, thus, are highly reactive. This can be highly damaging to humans and plants at the molecular level due to their possible interaction with nucleic acids, proteins, and lipids. These free radical interactions are damaging since they result in a whole chain of free radical reactions. More specifically, the interaction of an initial free radical (often reactive oxygen species) with another molecule changes that molecule itself into a free radical, which then reacts with other molecules, also turning them into free radicals. Ascorbate can terminate these chained radical reactions by serving as a stable (electron + proton) donor in interactions with free radicals, being converted into semidehydroascorbate and then dehydroascorbate. The net reaction is RO + C6H7O6- -> ROH + C6H6O6-. The oxidized forms of ascorbate are relatively unreactive, and do not cause cellular damage. They can be reversed back to ascorbate by cellular enzymes.
However, being a good electron donor, excess ascorbate in the presence of free metal ions can not only promote, but also initiate free radical reactions, thus making it a potentially dangerous pro-oxidative compound in certain metabolic contexts.
Uses
Ascorbic acid is easily oxidized and so is used as a reductant in photographic developer solutions (among others) and as a preservative.
Exposure to oxygen, metals, light, or heat destroys ascorbic acid, so it must be stored in a dark, cold, and non-metallic container.
The L-enantiomer of ascorbic acid is also known as vitamin C. The name "ascorbic" comes from its property of preventing and curing scurvy. Primates, including humans, and a few other species in all divisions of the animal kingdom, notably the guinea pig, have lost the ability to synthesize ascorbic acid, and must obtain it in their food.
Ascorbic acid and its sodium, potassium, and calcium salts are commonly used as antioxidant food additives. These compounds are water-soluble and thus cannot protect fats from oxidation: For this purpose, the fat-soluble esters of ascorbic acid with long-chain fatty acids (ascorbyl palmitate or ascorbyl stearate) can be used as food antioxidants. Eighty percent of the world's supply of ascorbic acid is produced in China.[4]
The relevant European food additive E numbers are
- E300 ascorbic acid,
- E301 sodium ascorbate,
- E302 calcium ascorbate,
- E303 potassium ascorbate,
- E304 fatty acid esters of ascorbic acid (i) ascorbyl palmitate (ii) ascorbyl stearate.
In plastic manufacturing, ascorbic acid can be used to assemble molecular chains more quickly and with less waste than traditional synthesis methods.[5]
Ascorbic acid synthesis in living organisms
Ascorbic acid is found in the biology of plants, animals, and single-cell organisms.[6] All animals either make it, eat it, or else die from scurvy due to lack of it. Reptiles and older orders of birds make ascorbic acid in their kidneys. Recent orders of birds and most mammals make ascorbic acid in their liver where the enzyme L-gulonolactone oxidase is required to convert glucose to ascorbic acid.[6] Humans, some other primates, and guinea pigs are not able to make L-gulonolactone oxidase because of a genetic mutation and are therefore unable to make ascorbic acid. Synthesis and signalling properties are still under investigation[7].
Compendial status
See also
- Colour retention agent
- Vitamin C: a discussion of the medical properties of ascorbic acid as well as its historic and social role
- Dehydroascorbic acid, an oxidized form of ascorbic acid.
- Erythorbic acid: a diastereomer of ascorbic acid.
- Mineral ascorbates: salts of ascorbic acid
- D-erythroascorbic acid: yeasts do not make vitamin C (L-ascorbic acid), but a similar antioxidant known as D-erythroascorbic acid
- Acids in wine
- Evolution of Vitamin C
Notes & references
- ↑ "Safety (MSDS) data for ascorbic acid". Oxford University. 2005-10-09. http://physchem.ox.ac.uk/MSDS/AS/ascorbic_acid.html. Retrieved 2007-02-21.
- ↑ Svirbelf, Joseph Louis; Szent-Gyorgyi, Albert (April 25, 1932), The Chemical Nature Of Vitamin C, http://profiles.nlm.nih.gov/WG/B/B/G/W/_/wgbbgw.pdf. Part of the National Library of Medicine collection. Accessed January 2007
- ↑ A website with an excerpt to using iodine
- ↑ Weiss, Rick (May 20, 2007), "Tainted Chinese Imports Common", Washington Post, http://www.washingtonpost.com/wp-dyn/content/article/2007/05/19/AR2007051901273.html, retrieved 2010-04-25.
- ↑ Vitamin C, water have benefits for plastic manufacturing, Reliable Plant Magazine, 2007, http://reliableplant.com/article.asp?pagetitle=Vitamin%20C,%20water%20have%20benefits%20for%20plastic%20manufacturing&articleid=3133, retrieved 2007-06-25.
- ↑ 6.0 6.1 Stone, Irwin (1972), The Natural History of Ascorbic Acid in the Evolution of Mammals and Primates, http://www.seanet.com/~alexs/ascorbate/197x/stone-i-orthomol_psych-1972-v1-n2-3-p82.htm.
- ↑ Valpuesta, Victoriano; Botella, Miguel (December 2004), "Biosynthesis of L-ascorbic acid in plants: new pathways for an old antioxidant", TRENDS in Plant Science 9 (12), http://www.bmbq.uma.es/lbbv/index_archivos/pdf/Valpuesta%202004.pdf
- ↑ British Pharmacopoeia Commission Secretariat (2009). "Index, BP 2009". http://www.pharmacopoeia.co.uk/pdf/2009_index.pdf. Retrieved 4 February 2010.
- ↑ "Japanese Pharmacopoeia, Fifteenth Edition". 2006. http://jpdb.nihs.go.jp/jp15e/JP15.pdf. Retrieved 4 Februally 2010.
Further reading
- Clayden; Greeves; Warren; Wothers (2001), Organic Chemistry, Oxford University Press, ISBN 0-19-850346-6.
- Davies, Michael B.; Austin, John; Partridge, David A., Vitamin C: Its Chemistry and Biochemistry, Royal Society of Chemistry, ISBN 0-85186-333-7.
- Coultate, T. P., Food: The Chemistry of Its Components (3rd ed.), Royal Society of Chemistry, ISBN 0-85404-513-9.
- Gruenwald, J.; Brendler, T.; Jaenicke, C., eds. (2004), PDR for Herbal Medicines (3rd ed.), Montvale, NJ: Thomson PDR.
External links
- International Chemical Safety Card 0379
- SIDS Initial Assessment Report for L-Ascorbic acid from the Organisation for Economic Co-operation and Development (OECD)
- IPCS Poisons Information Monograph (PIM) 046
- Safety data from the University of Oxford.
- The Effect of Ascorbic Acid on Bleaching Coral
- The Effect of Ascorbic Acid on Fragmented Coral
- Interactive 3D-structure of vitamin C with details on the x-ray structure
|
||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||
|
|||||